-
PDF
- Split View
-
Views
-
Cite
Cite
Jan Heuschele, Thomas Kiørboe, The smell of virgins: mating status of females affects male swimming behaviour in Oithona davisae, Journal of Plankton Research, Volume 34, Issue 11, November 2012, Pages 929–935, https://doi.org/10.1093/plankt/fbs054
Close - Share Icon Share
Abstract
Many copepod species rely on pheromone cues to find partners. Some parasitic and benthic copepod males are able to distinguish between females of different reproductive states. Here, we demonstrate that the swimming activity and velocity of males of a pelagic copepod, Oithona davisae, increases in the presence of virgin when compared with mated females and that the cue is waterborne. The ability to distinguish between virgin and mated females may reduce male mortality during mate search and the cost related to mating behaviour (precopula) in both sexes. We estimate that at realistic population densities the ability of males to distinguish between virgin and mated females saves them several hours per day of dangerous and energetically expensive fast female tracking.
Introduction
Sexually reproducing organisms have to find and identify potential partners to be able to produce offspring. Pelagic copepods and other planktonic organisms live in a vast three-dimensional space of the open ocean, a physical regime dominated by low Reynolds numbers. The habitat, the organisms’ size, and the fact that the number of potential mates is low and varies with season complicates mate finding for these organisms (Mauchline, 1998). Copepods use chemical and hydro-chemical signals to find food and mating partners, as well as to avoid predation (Tsuda and Miller, 1998; Ingvarsdottir et al., 2002). Several species use female pheromones to track and find mating partners (Katona, 1973; Doall et al., 1998; Lonsdale et al., 1998). Chemical signals persist longer than hydro-mechanical signals that dissipate fast in a low Reynolds number regime (Yen et al., 1998). The use of pheromone trails extends the females’ signal by up to 100 times her body size (Yen and Lasley, 2010). Unfortunately, not much is known about the chemical composition of copepod pheromones, but research on salmon lice (Lepeophtheirus salmonis) indicates that the pheromones consist of small, lipophilic organic molecules (Ingvarsdottir et al., 2002).
In pelagic copepods, pheromone signals are sought after and followed by males, and males often swim faster and with more directional persistence than females (Kiørboe and Bagøien, 2005). Males encountering an odour signal typically start to track down the emitting female (Kiørboe, 2007). Male tracking behaviour can be distinguished from searching and foraging behaviour due to dramatically increased swimming velocities and altered swimming trajectories (Bagøien and Kiørboe, 2005; Kiørboe, 2007). The resulting enlarged hydro-mechanical foot print and increased encounter rate with predators during both mate tracking and mate searching potentially make males more vulnerable to predation than females. That is an important factor in causing the skewed sex ratios of copepod populations (Hirst et al., 2010).
The release of pheromones is potentially costly for females. Although the production of pheromones is likely to be cheap compared with other metabolic processes (Bagøien and Kiørboe, 2005), the release of pheromones may increase the detectability by predators. In addition, the coupling of male and female during precopula makes both of them more susceptible to visual predators (Jersabek et al., 2007), especially in species in which precopula lasts for a long time (Maier, 1996). Hence, in addition to increasing mate encounter rates, emission of pheromones may also imply elevated mortality. Females could reduce the costs of mating if they were to only attract males when they reach maturity or have emptied their spermathecae, and males would likewise benefit from only tracking receptive females.
Mated and unmated females have different chemical odour profiles in many organisms and in many species mate searching males are able to distinguish these two states (Thomas, 2010). The determination of the mating status of a potential partner can involve contact or diffusible pheromones (Kelly et al., 1998). In the parasitic copepod Lepeophtheirus salmonis, adult males showed directional responses to pre-adult virgins and to water preconditioned with pre-adult virgins but not to mated females, (Ingvarsdottir et al., 2002), indicating either that males are able to distinguish the smell from virgins and mated females, or that mated females stop emitting pheromones. In the freshwater copepods, Diaptomus minutus and Diaptomus oregonensis the males preferred mating with females with mature ovaries over those that were not ready for mating (Chow-Fraser and Maly, 1988). Males of Oithona davisae, seem to mate more often with unmated females (Uchima, 2005) and there is anecdotal evidence that males show different swimming behaviours when confronted with mated and virgin females (Uchima and Murano, 1988). Oithona davisae is an ambush feeder and males have to sacrifice feeding when searching for females (Kiørboe, 2007). The consequential reduced feeding rate and the elevated risk of encountering predators during mate search may explain the much higher mortality of males than females and the strongly female biased adult sex ratios in field populations of this species (Uye and Sano, 1995; Kiørboe, 2007). Hence, the ability to detect the presence of perceptive females and to distinguish between unmated and mated females may drastically reduce mate mortality.
In this study, we compare the swimming behaviour of males in the presence of virgin and mated females and examine the ability of males of O. davisae to assess the mating status of potential partners.
Method
Experimental animals
Oithona davisae is a small cyclopoid copepod (∼0.3 mm prosome length). Small cyclopoids comprise most of the planktonic biomass and play a major role in the marine food web (Gallienne and Robins, 2001; Turner, 2004). Oithona davisae lives in coastal and estuarine environments with typical summer adult densities between 10 and 500 L−1 (Uye and Sano, 1995). It is known that the males find females using chemical cues emitted by the female (Uchima and Murano, 1988). Laboratory stock populations of O. davisae were kept at a temperature of 20°C and fed at libitum with the dinoflagellate Oxyrrhis marina. We incubated late copepodites individually in 24-well plates in 3 mL of filtered seawater. Every other day 1 mL of water was exchanged and O. marina added at a saturating concentration (>500 μg C L−1, Ceballos and Kiørboe, 2011). The wells were checked daily for freshly matured individuals. These were then used in the experiments.
Individual virgin females were placed in 4 mL of seawater with a male. Every half an hour we examined the females for attached spermatophores. We assumed that the presence of at least one spermatophore indicates a successful mating event. Mated females were immediately transferred individually to a 70 mL cell culture bottle with 40 mL of seawater with a concentration of 15 O. marina cells mL−1. For each mated female, we simultaneously transferred a virgin female and in most cases a virgin male individually to two other cell culture bottles. These individuals were then incubated for 24 h to produce scent.
After this period 10 adult males were pipetted into each bottle with a minimum amount of water, resulting in a total number of 11 individuals per bottle. These males had been isolated 24 h earlier from the stock population, and kept in groups of 5 in darkness at the same temperature and food concentration as the scent-producing animals. 24 h is sufficient time for males to rebuild their spermatophores (Ceballos and Kiørboe, 2011). We did not remove the scent producing animal to reduce the perturbation of the pheromone patches while trying to catch this individual.
Video setup and analysis
Within 1 min after the addition of the 10 males we started a 10-min recording of swimming behaviour of all individuals. We placed the bottle in front of a collimated infrared light source and video-recorded shadow images of the copepods at a frame rate of 25 Hz with a black-and-white CCD, equipped with a 35-mm lens (Kiørboe, 2007). The field of view was 3.5 × 4.0 cm2. Five-minute sequences (minutes 1–6) were analysed for copepod swimming activity. The first minute was skipped to minimize the possibility that swimming trajectories were influenced by transferring the males and switching of the light in the experimental room. Swimming trajectories were recorded by automatically tracking all individual copepods in the movies using LabTrack software (BioRas; Kiørboe and Bagøien, 2005). The software identifies the (X,Y) position of all copepods at each video frame, which was used as a basis for further analyses (see below). The video analysis also included the scent-producing copepods; ideally, these should have been omitted from the analyses. The addition of males to the experimental bottles and the analysis of the video sequences were done without the knowledge of the treatment.
Statistical analysis
In total 44 bottles were filmed and analyzed; 14 with virgin females, 17 with mated females, and 13 with virgin males. We used the number of recorded swimming trajectories as a measure of overall swimming activity of the copepods. The number of trajectories (N) was log-transformed, and differences in the number of tracks were analyzed using a linear model with treatment as independent factor and the number of tracks as dependent variable (Fig. 1). The analysis of the characteristics (velocity, directional persistence) of the swimming trajectories was restricted to the first 3 s of each individual trajectory to ensure that enough trajectories contributed to the calculation and to reduce confounding effects when animals reach the border of the field of view.
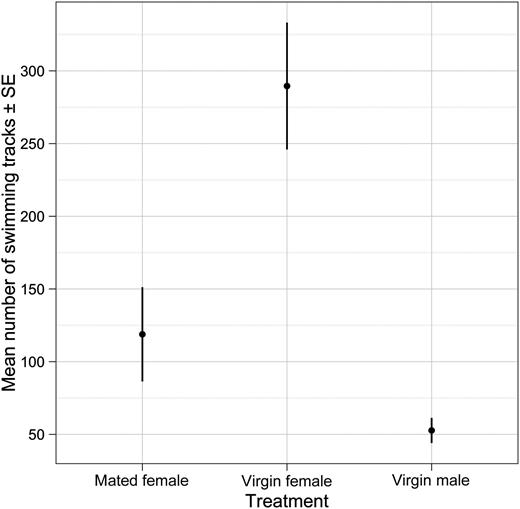
Male swimming activity, quantified by the average number ± SE of trajectories per 5 min of observation, in the presence of a virgin female, a mated females and a male. The “virgin female” treatment differed significantly from the two other treatments (F2,41 = 9.91, P = 0.00031); treatments “mated females” and “males” did not differ significantly (P = 0.49).
Differences in the swimming velocities were used to distinguish between ambush feeding (∼ zero velocity), mate search and female pheromone tracking behaviour. Mate search velocities in O. davisae average 15 ± 6 mm s−1 and velocities that exceed 40 mm s−1 identify males that follow female pheromone trails consisting of distinct pheromone patches (Kiørboe, 2007). We refer to these as tracking events. To test for difference in the occurrence of tracking events, we used a generalized linear model with a binomial link function and replicate as the random factor. Differences in the frequency distributions of swimming velocities between treatments were tested using a Kruskal–Wallis rank sum test (Fig. 2). We analysed the difference in the average swimming velocity per recorded trajectory using a linear mixed model with treatment as independent factor, log transformed swimming velocity as dependent and replicate as random factor.
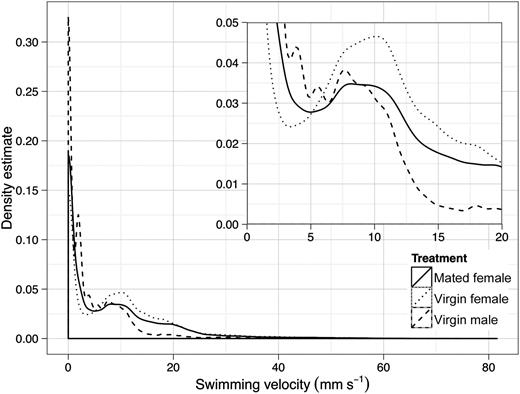
Swimming velocity distribution of copepods for each treatment plotted as kernel density estimates (overview over all recorded velocities and detailed view of the slower velocities) (Kruskal–Wallis χ2 = 9146.6, df = 2, P < 0.001). The velocity measurements were made at 25 Hz.
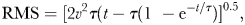
To investigate whether the increased swimming activity resulted in longer travelled distances, we compared the gross swimming distances between treatments. We used a linear mixed model with treatment as fixed, log transformed travelled distance as dependent and replicate as a random factor.
All post hoc tests between the single treatments were done using the glht-function (Hothorn et al., 2008) for multiple comparisons of the mean using Tukey contrasts. We used the statistical software R version 2.13.1 for all analysis (R Development Core Team, 2011), with the packages “lmer” for generalized mixed models and “lme” for linear mixed models.
Results
Swimming activity and velocity
Males showed a significantly higher swimming activity in water conditioned by a virgin female compared with the two other treatments, while the swimming activity in the presence of mated females and males did not differ significantly (Fig. 1).
Males in the “virgin female” treatment swam faster than in the “male” treatment (F2,41 = 16.46, P < 0.001), while male swimming velocities in the “male” and “mated female” treatments were indistinguishable (P = 0.325, Table I).
Statistics of copepod swimming trajectories characteristics
| Treatment . | Total number of analysed swimming trajectories . | Average swimming velocity v (mm s−1) . | Decorrelation length scale λ (mm) . | Average number of swimming trajectories (min−1)a . | Average number of tracking events (min−1)b . |
|---|---|---|---|---|---|
| Virgin female | 4054 | 9.13 | 4.49 | 57.91 | 24.77 |
| Mated female | 2020 | 7.64 | 1.87 | 23.76 | 9.22 |
| Virgin male | 685 | 4.15 | 1.95 | 10.54 | 2.23 |
| Treatment . | Total number of analysed swimming trajectories . | Average swimming velocity v (mm s−1) . | Decorrelation length scale λ (mm) . | Average number of swimming trajectories (min−1)a . | Average number of tracking events (min−1)b . |
|---|---|---|---|---|---|
| Virgin female | 4054 | 9.13 | 4.49 | 57.91 | 24.77 |
| Mated female | 2020 | 7.64 | 1.87 | 23.76 | 9.22 |
| Virgin male | 685 | 4.15 | 1.95 | 10.54 | 2.23 |
aThe number of recorded trajectories is higher in the virgin female treatment compared with the two others (F2,41 = 13.67, P < 0.0001), but not between mated females and virgin males (P = 0.29).
bThe number of tracking events (trajectories including velocities v > 40 mm s−1) was higher in virgin female treatment compared with the two other treatments (VirginF – MatedF z = 2.78, P = 0.015, VirginF – VirginM z = −3.544, P = 0.0011), but not between VirginM and MatedF treatment (P = 0.57).
Statistics of copepod swimming trajectories characteristics
| Treatment . | Total number of analysed swimming trajectories . | Average swimming velocity v (mm s−1) . | Decorrelation length scale λ (mm) . | Average number of swimming trajectories (min−1)a . | Average number of tracking events (min−1)b . |
|---|---|---|---|---|---|
| Virgin female | 4054 | 9.13 | 4.49 | 57.91 | 24.77 |
| Mated female | 2020 | 7.64 | 1.87 | 23.76 | 9.22 |
| Virgin male | 685 | 4.15 | 1.95 | 10.54 | 2.23 |
| Treatment . | Total number of analysed swimming trajectories . | Average swimming velocity v (mm s−1) . | Decorrelation length scale λ (mm) . | Average number of swimming trajectories (min−1)a . | Average number of tracking events (min−1)b . |
|---|---|---|---|---|---|
| Virgin female | 4054 | 9.13 | 4.49 | 57.91 | 24.77 |
| Mated female | 2020 | 7.64 | 1.87 | 23.76 | 9.22 |
| Virgin male | 685 | 4.15 | 1.95 | 10.54 | 2.23 |
aThe number of recorded trajectories is higher in the virgin female treatment compared with the two others (F2,41 = 13.67, P < 0.0001), but not between mated females and virgin males (P = 0.29).
bThe number of tracking events (trajectories including velocities v > 40 mm s−1) was higher in virgin female treatment compared with the two other treatments (VirginF – MatedF z = 2.78, P = 0.015, VirginF – VirginM z = −3.544, P = 0.0011), but not between VirginM and MatedF treatment (P = 0.57).
The distribution of swimming velocities similarly differed statistically and again with only the “virgin female” treatment significantly different from the others (Fig. 2). The distribution of swimming speeds shows that slow swimming and pauses dominated the majority of trajectories in all treatments: this corresponds to ambush feeding individuals. In the “virgin male” treatment, there were relatively few instances of higher swimming speeds, while in the “mated female” and particularly in the “virgin female” treatment, events with speeds ≥10 mm s−1 were more common. This corresponds to mate searching behaviour. Moreover, fast swimming bursts with speeds of >40 mm s−1, corresponding to males following female pheromone tracks, were significantly more common in the “virgin female” treatment (see Table I).
Motility pattern
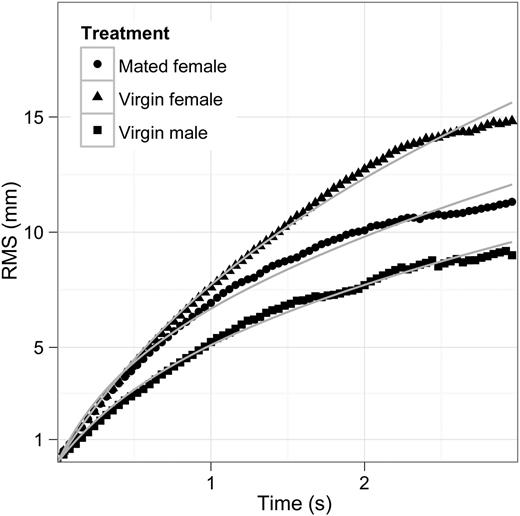
Motility patterns were different between the treatments: The directional persistence, quantified by the decorrelation length scale, was highest in the “virgin female” treatment and lowest in the “male” treatment (Table I, Fig. 3). The combination of high swimming velocity and higher directional persistence, lead to a larger net distance travelled by males in the virgin female treatments compared with the two other treatments (Fig. 3). These males therefore have a higher chance of meeting a female, but also a higher risk of encountering a predator.
Differences in the root mean square distance travelled between all three treatments (restricted to the first 3 s). The travelled net distance was significantly longer in both female treatments compared with the male treatment (F2,41 = 12.05, P = 0.0001), but not between them (P = 0.69).
The differences in swimming velocity resulted in a distinct difference in gross distances travelled per recorded swimming trajectory (F2,41 = 14.98, P < 0.001). The travelled distance was significantly longer in the virgin female treatment compared with the two other treatments, but not between mated females and virgin males (P = 0.064).
Discussion
The presence of virgin O. davisae females elicited an increased mate search behaviour and the occurrence of female pheromone tracking events in the males compared with the presence of mated females or virgin males. There were no big differences between the reactions of males towards the odour of a virgin male or mated female. This change in behaviour must be mediated through a waterborne, chemical cue. It has previously been demonstrated that mate-tracking behaviour is elicited by distinct odour patches in the vicinity of females (Uchima and Murano, 1988; Kiørboe, 2007). We have here in addition demonstrated that background concentrations of signal molecules produced by virgin females elicit intensified mate searching behaviour. This indicates either (i) that females only produce chemical signals that attract males when they are receptive, or (ii) that males can distinguish between the chemical profile of virgin and fertilized females. During the last decade a few studies have tried to extract and characterize copepod pheromones, but so far without success. Research on salmon lice (L. salmonis) indicates that the pheromones consist of small, lipophilic organic molecules (Ingvarsdottir et al., 2002). Biological knowledge about the release and perception of pheromones is a necessary requisite for successful extraction of pheromone molecules for subsequent identification. This will reduce potential chemical noise from other sources. Using virgin animals will therefore increase the chances of extracting a sufficient amount of female pheromone molecules, and to reduce the noise in the signal in the sampled exudates, at least in O. davisae and most likely in other species of copepods with a similar mating pattern.
It is known from other animals that individuals have a timed release of pheromones. In the swordtail fish (Xiphophorus birchmanni), for example, males increase the delivery of pheromones in the presence of females (Rosenthal et al., 2011) and females of Chinese hermit crabs seem to emit pheromone only once a male has made physical contact with her (Herborg et al., 2006). Lonsdale et al. (Lonsdale et al., 1998) already suggested that pheromone attraction in copepods may stop after copulation. This could be controlled by the mating behaviour of males. The placement of the spermatophore may prevent the release of pheromones through the genital opening (Vigoni et al. 1999). In several copepod species, the placement of the spermatophore involves sealing the genial atrium by a “mating plug” which rests in place even after the spermatophore is removed (Anstensrud, 1990; Corni et al., 2001; Barthélémy et al., 2003). We do not know, however, from where pheromones are actually released in copepods.
If the release is continuous, such as would be the case if the pheromones are by-products of metabolism (Yen and Lasley, 2010), males must be able to detect changes in the chemical composition of the female cues (Watras, 1983). Males in several copepod species are able to distinguish between virgin and inseminated females, and normally prefer to mate with the former (Uchima and Murano, 1988; Lazzaretto et al., 1993). The identification of the females mating status seems to be sometimes mediated by contact pheromones (Ting et al., 2000). It is unknown, although likely, whether the same is true for diffusible pheromones. The mechanisms behind potential changes of the chemical profiles and their detection are still unknown for most species, as is the chemical identity of the pheromones (Thomas, 2010).
Males of O. davisae did not show any increased activity towards other males which contrasts with the findings for Temora longicornis of Yen and Lasley (Yen and Lasley, 2010). This suggests a difference in the mating tactics and the usage of pheromones between these two species. Females of T. longicornis lack seminal receptacles (Barthélémy et al., 1998) and are believed to depend on multiple matings due to limited sperm storing capacity [but see Sichlau and Kiørboe (Sichlau and Kiørboe, 2011)], while O. davisae females can store sperm and apparently only need to mate once during the lifetime (Ceballos and Kiørboe, 2011). Although cases of multiple matings are reported in this species (Uchima, 1985), they do not lead to increased production of fertile eggs (Ceballos and Kiørboe, 2011). Hence, males of especially this species are likely to have decreased benefits from mating with an inseminated female, and it would be beneficial to focus the search on virgin, mature females. In T. longicornis males may still have the ability to inseminate some of the eggs of a previously mated female.
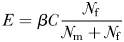
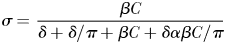
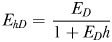
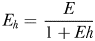
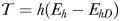
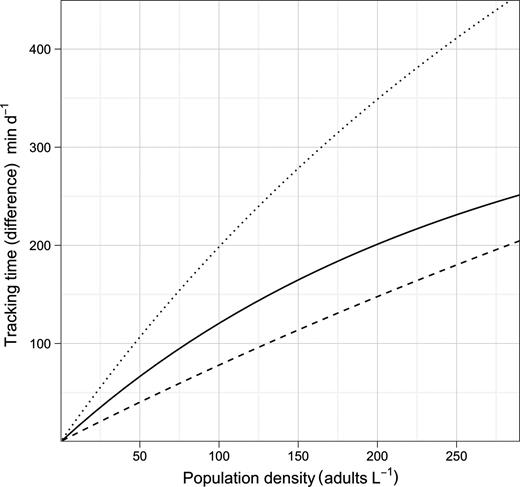
From equations (1)–(4), it follows that at realistic adult densities, the ability to distinguish between mated and virgin (receptive) females may save the males several hours per day of dangerous and energetically expensive fast female tracking (Fig. 4). A male that searches and tracks mated females may, thus, experience elevated mortality, and it may waste a spermatophore on an already mated female and, hence, loose future mating opportunities. Male copepods have rather limited reproductive capacity and O. davisae can only fertilize <2 females daily during a period of <10 days (Ceballos and Kiørboe, 2011). Finally, time used on fruitless mate searching detracts from feeding time, because an ambush feeding copepod cannot feed and search for mates simultaneously. Thus, the selective pressure for developing an ability to evaluate females remotely must be substantial.
The daily duration of female tracking and copulation behaviour of males that can distinguish between virgin and mated females (dashed line), and those who cannot (dotted line), as well as the time difference between both types (solid line) as a function of adult density. Equations (1)–(3) were used for the calculations, and parameter values taken from Kiørboe (Kiørboe, 2006, 2007) were: α = 1.1 day, β = 102.01PL3 L d−1 with PL = 0.3 mm (prosome length), δ = 0.1 d−1, sex ratio π= 0.2 and a general handling time h of 1 min. The range of population assumed is realistic (Uye and Sano, 1995).
FUNDING
This work was supported by the German Science Foundation DFG by the (grant number He 6050-1/1 to J.H.).
ACKNOWLEDGEMENTS
We would like to thank J. Sainmont for discussions, and J. Melbye for maintenance of the stock cultures.
REFERENCES
Author notes
Corresponding editor: Marja Koski